7月8日,发生了一件非常奇怪的事情:制药巨头渤健(Biogen)主动要求美国食品与药品监督管理局(FDA)缩窄一种药物的处方标签范围,也就是说,他们削减了自己的市场范围。这种药物正是这家公司6月7日获批的,颇具开创性和争议性的阿尔茨海默病药物Aduhelm(其科学药名为阿杜那单抗)。
批准该药物用于治疗大多数阿尔茨海默病患者的FDA,在渤健提出申请后予以了批准。不过一些细节和官方变更还有待最终商定。
渤健在申请中强调,Aduhelm上市之初应当用于有轻度认知障碍或轻度痴呆症状的患者,因为此类患者参与过Aduhelm获批的临床研究。
应当注意的是,目前尚不清楚究竟是FDA,还是渤健发起的标签变更流程。但无论是谁,最终结果都是一样的。
大型药企通常不会主动拒绝一项具有里程碑意义的疗法可能带来的数十亿美元潜在收入。不过,考虑到Aduhelm高昂的价格和阿尔茨海默病在美国的普遍程度,即便缩减了市场范围,渤健依然可以通过Aduhelm获得巨大的经济收益。
但是,在Aduhelm获批的道路上,充满了坎坷,遭遇了颇为严苛的审查。而且,即使改变了处方标签,医生和希望使用渤健药物的患者之间的关系可能很快也会变得非常尴尬。
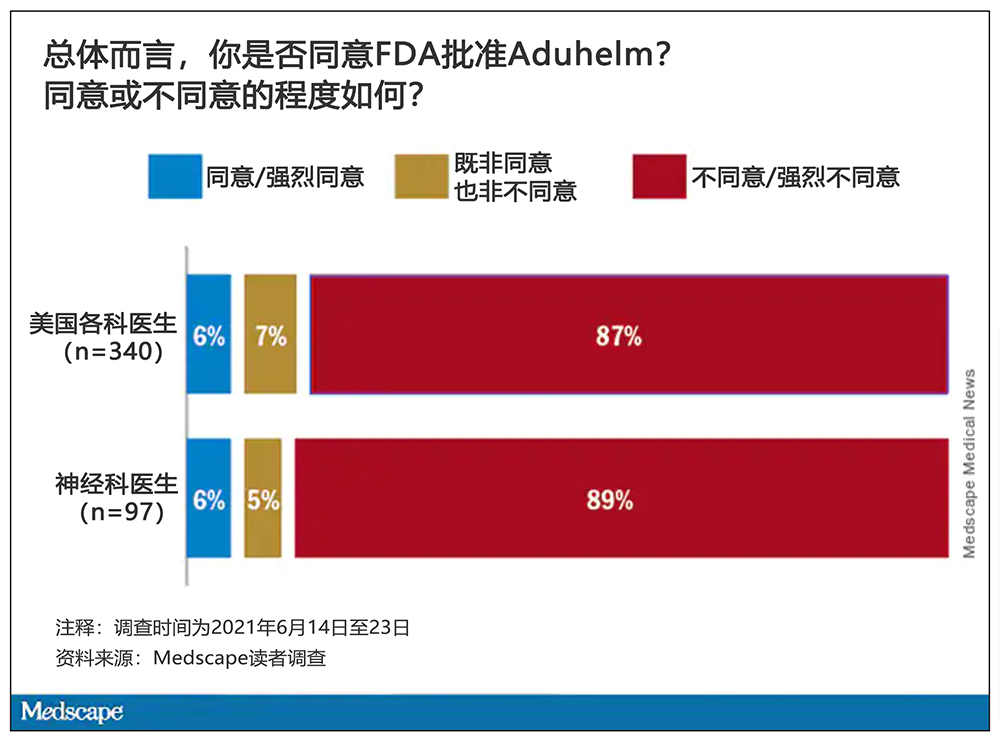
一项由医疗保健刊物Medscape开展的新调查显示,绝大多数医生,包括89%的神经学家,都对批准Aduhelm的决定持不同意或强烈不同意意见。因为几乎没有证据表明该药物可以延缓阿尔茨海默病患者认知能力的下降。
也就是说,如果患者家属执着地想要找到一种可行的疗法,除掉阿尔茨海默病的病根,而非只是抑制其表面症状,那么他们可能会和不愿意给他们开这种药物的医生发生口角冲突。
此前,尽管FDA自己的独立专家小组竭力反对,但该机构还是批准了将Aduhelm广泛用于治疗阿尔茨海默病这一导致美国人死亡的主要疾病。
即便暂且不提7月8日的标签变更公告,Aduhelm获批一事也已经让FDA卷入了政治风暴。渤健尚未决定将Aduhelm的年定价确定为5.6万美元——如果该药物价格最终定为这一数字,那么哪怕只有100万受益人使用此药物,联邦医保等公共医保计划每年也要为此花费570亿美元。
此外,尽管渤健有近十年的时间来证明目前被批准的这一疗法在实际应用中是否有效,但公司仅承诺Aduhelm四年内不涨价。
Medscape的调查显示,这一系列戏剧性的事件始终萦绕在医生们的脑海之中。最大的问题似乎是,如果医生给阿尔茨海默病患者开出Aduhelm这样昂贵的治疗方案,但实际上药物却未能发挥作用,那么这只会给患者家属和照料者带来虚假的希望。
考虑到这一情况,医生为患者开Aduhelm是否合理?约60%参与调查的医生表示,渤健的数据不足以清晰证明Aduhelm能够为患者带来明确的临床益处。另有79%参与调查的美国医生和68%参与调查的神经科医生称,他们不打算为阿尔茨海默病患者开Aduhelm。
美国医学界这种有所保留的态度,最终可能还是会屈服于病人对这一药物的支持,Aduhelm最终出人意料获批的很大一部分原因也正在于此。
不过,美国国会已经注意到了Aduhelm引发的越来越多争议。西弗吉尼亚州参议员乔•曼钦、新泽西州众议员弗兰克•帕隆、纽约州众议员卡罗琳•马洛尼和加州众议员凯蒂•波特等人,都呼吁对FDA展开更加严格的审查。
这可能会导致公众呼吁FDA领导层改组(这也正是曼钦呼吁的),要求彻查FDA和其负责监管的制药行业之间,是否存在一些监督组织所指出的暧昧关系。
美国众议院监督和改革委员会主席马洛尼、众议院能源和商业委员会主席帕隆于今年6月在一份声明中写道:“委员会将会调查这一问题,以便国会和美国人民更好地了解这一药物的获批原因、定价,以及它未来对阿尔茨海默病疗法研究和联邦医保计划产生怎样的影响。”
波特以对包括大型制药公司高管在内的国会证人的严格务实的调查而闻名。7月6日,她加入了这场争论,并呼吁对FDA官员与渤健等制药公司打交道的方式展开独立调查。
患者应该相信,他们的治疗方案是有科学依据的,而不是建立在大型制药公司和FDA官员亲密关系之上的。我担心此前某些药物的审批流程存在猫腻,所以我敦促独立监督组织对此展开调查。
——众议员凯蒂•波特(@RepKatiePorter),2021年7月6日
据称,渤健自己的独立临床试验安全监督委员会认为Aduhelm“无效”,但公司试图游说FDA官员让该药物通过最终监管审批。STAT News的一篇报道指出,渤健和FDA高级工作人员进行了多次非公开的秘密会议,公司似乎决心要将Aduhelm推向市场。
Aduhelm的经济和监管前景目前尚不明朗。但是如今,医生的质疑声和政客的愤怒指责声四起,正是因为该药物的审批流程存在一些不清不楚的地方,这一所谓开创性阿尔茨海默病药物才会引发眼下混乱的争议。(财富中文网)
译者:钱功毅
7月8日,发生了一件非常奇怪的事情:制药巨头渤健(Biogen)主动要求美国食品与药品监督管理局(FDA)缩窄一种药物的处方标签范围,也就是说,他们削减了自己的市场范围。这种药物正是这家公司6月7日获批的,颇具开创性和争议性的阿尔茨海默病药物Aduhelm(其科学药名为阿杜那单抗)。
批准该药物用于治疗大多数阿尔茨海默病患者的FDA,在渤健提出申请后予以了批准。不过一些细节和官方变更还有待最终商定。
渤健在申请中强调,Aduhelm上市之初应当用于有轻度认知障碍或轻度痴呆症状的患者,因为此类患者参与过Aduhelm获批的临床研究。
应当注意的是,目前尚不清楚究竟是FDA,还是渤健发起的标签变更流程。但无论是谁,最终结果都是一样的。
大型药企通常不会主动拒绝一项具有里程碑意义的疗法可能带来的数十亿美元潜在收入。不过,考虑到Aduhelm高昂的价格和阿尔茨海默病在美国的普遍程度,即便缩减了市场范围,渤健依然可以通过Aduhelm获得巨大的经济收益。
但是,在Aduhelm获批的道路上,充满了坎坷,遭遇了颇为严苛的审查。而且,即使改变了处方标签,医生和希望使用渤健药物的患者之间的关系可能很快也会变得非常尴尬。
一项由医疗保健刊物Medscape开展的新调查显示,绝大多数医生,包括89%的神经学家,都对批准Aduhelm的决定持不同意或强烈不同意意见。因为几乎没有证据表明该药物可以延缓阿尔茨海默病患者认知能力的下降。
也就是说,如果患者家属执着地想要找到一种可行的疗法,除掉阿尔茨海默病的病根,而非只是抑制其表面症状,那么他们可能会和不愿意给他们开这种药物的医生发生口角冲突。
此前,尽管FDA自己的独立专家小组竭力反对,但该机构还是批准了将Aduhelm广泛用于治疗阿尔茨海默病这一导致美国人死亡的主要疾病。
即便暂且不提7月8日的标签变更公告,Aduhelm获批一事也已经让FDA卷入了政治风暴。渤健尚未决定将Aduhelm的年定价确定为5.6万美元——如果该药物价格最终定为这一数字,那么哪怕只有100万受益人使用此药物,联邦医保等公共医保计划每年也要为此花费570亿美元。
此外,尽管渤健有近十年的时间来证明目前被批准的这一疗法在实际应用中是否有效,但公司仅承诺Aduhelm四年内不涨价。
Medscape的调查显示,这一系列戏剧性的事件始终萦绕在医生们的脑海之中。最大的问题似乎是,如果医生给阿尔茨海默病患者开出Aduhelm这样昂贵的治疗方案,但实际上药物却未能发挥作用,那么这只会给患者家属和照料者带来虚假的希望。
考虑到这一情况,医生为患者开Aduhelm是否合理?约60%参与调查的医生表示,渤健的数据不足以清晰证明Aduhelm能够为患者带来明确的临床益处。另有79%参与调查的美国医生和68%参与调查的神经科医生称,他们不打算为阿尔茨海默病患者开Aduhelm。
美国医学界这种有所保留的态度,最终可能还是会屈服于病人对这一药物的支持,Aduhelm最终出人意料获批的很大一部分原因也正在于此。
不过,美国国会已经注意到了Aduhelm引发的越来越多争议。西弗吉尼亚州参议员乔•曼钦、新泽西州众议员弗兰克•帕隆、纽约州众议员卡罗琳•马洛尼和加州众议员凯蒂•波特等人,都呼吁对FDA展开更加严格的审查。
这可能会导致公众呼吁FDA领导层改组(这也正是曼钦呼吁的),要求彻查FDA和其负责监管的制药行业之间,是否存在一些监督组织所指出的暧昧关系。
美国众议院监督和改革委员会主席马洛尼、众议院能源和商业委员会主席帕隆于今年6月在一份声明中写道:“委员会将会调查这一问题,以便国会和美国人民更好地了解这一药物的获批原因、定价,以及它未来对阿尔茨海默病疗法研究和联邦医保计划产生怎样的影响。”
波特以对包括大型制药公司高管在内的国会证人的严格务实的调查而闻名。7月6日,她加入了这场争论,并呼吁对FDA官员与渤健等制药公司打交道的方式展开独立调查。
患者应该相信,他们的治疗方案是有科学依据的,而不是建立在大型制药公司和FDA官员亲密关系之上的。我担心此前某些药物的审批流程存在猫腻,所以我敦促独立监督组织对此展开调查。
——众议员凯蒂•波特(@RepKatiePorter),2021年7月6日
据称,渤健自己的独立临床试验安全监督委员会认为Aduhelm“无效”,但公司试图游说FDA官员让该药物通过最终监管审批。STAT News的一篇报道指出,渤健和FDA高级工作人员进行了多次非公开的秘密会议,公司似乎决心要将Aduhelm推向市场。
Aduhelm的经济和监管前景目前尚不明朗。但是如今,医生的质疑声和政客的愤怒指责声四起,正是因为该药物的审批流程存在一些不清不楚的地方,这一所谓开创性阿尔茨海默病药物才会引发眼下混乱的争议。(财富中文网)
译者:钱功毅
A truly strange thing happened on July 8: Pharma giant Biogen, maker of the pioneering and highly controversial Alzheimer's drug Aduhelm (also called by its scientific name aducanumab), voluntarily hacked away at its own market reach by requesting that the Food and Drug Administration (FDA) cut back on the drug's prescribing label.
The agency, which had essentially approved Aduhelm for the vast majority of Alzheimer's patients, granted the request (although some details and official changes still need to be hammered out), wherein Biogen emphasized that Aduhelm should be initially funneled to patients with mild cognitive impairment or mild dementia since those were the kinds of patients involved in the clinical studies leading to the aducanumab approval. It should be noted that it's unclear whether the FDA or Biogen initiated the label changing process, but the end result is the same.
Drug giants don't typically cut off billions in potential revenues from a landmark treatment voluntarily, although the company could still reap massive financial gains from Aduhelm, given its hefty price tag and Alzheimer's prevalence in America. But nothing in the messy path to Aduhelm's approval and the fierce scrutiny it has elicited is exactly normal. And, even with the prescribing label change, things may soon become very awkward between doctors and their patients who are seeking Biogen's therapy.
A new survey conducted by Medscape, a health care-focused publication which caters to the medical profession, finds that an overwhelming number of physicians, including 89% of neurologists, either disagree or strongly disagree with the FDA's decision to approve Aduhelm despite little-to-no proof of its efficacy in slowing cognitive decline in Alzheimer's patients. And that means families clinging to the hope of finally having an available treatment that addresses the underlying disease of Alzheimer's, rather than just its symptoms, could be in for some tough conversations with doctors reluctant to prescribe it.
The fact that the FDA delivered a wide-ranging drug approval for a disease which is among the leading causes of death in America despite the agency's own expert independent panels' strong disapproval certainly hasn't quelled the political firestorm facing the regulatory body, July 8's label change announcement aside. Neither has Biogen's decision to set a yearly list price of $56,000 on aducanumab, which could cost public health programs such as Medicare $57 billion in a single year if just one million beneficiaries were to receive it. Plus, Biogen has only committed to four years of a moratorium on Aduhelm price increases even though it has close to a decade to demonstrate whether its now-approved treatment actually works in a real-world setting.
That series of dramatic events seems to be swirling in physicians' heads, per Medscape's survey. But the biggest issue appears to be whether or not it's justifiable to prescribe Alzheimer's patients a pricey treatment like Aduhelm only to give their families and caretakers false hope if it winds up flopping on a practical level. Some 60% of the physicians who responded to the poll said Biogen's data was unclear in establishing a clear clinical benefit for patients. And 79% of American physicians and 68% of neurologists who were surveyed say they don't plan to prescribe aducanumab to their Alzheimer's patients.
The seeming reticence among the U.S. medical class may eventually give way to patient advocacy pressure, which was largely credited with Aduhelm's unlikely approval as well. But Congress has already taken note of the growing controversy and the likes of Sen. Joe Manchin of West Virginia and Reps. Frank Pallone, Carolyn Maloney, and Katie Porter of New Jersey, New York, and California, respectively, have called for greater scrutiny of the FDA. That could lead to a public cry for a shakeup of FDA leadership, as Manchin has done, and an examination of what some watchdogs say has been a cozy relationship between the agency and the pharmaceutical industry it's charged with regulating.
"Our Committees will be investigating this matter so Congress and the American people can better understand why this drug was approved, how Biogen set its price and what impact this will have on research for future Alzheimer’s treatments and federal health care programs," wrote Reps. Maloney and Pallone, chairs of the respective House Oversight and Reform Committee and Energy and Commerce Committee, in a statement in June.
Porter, who is known for her hard-nosed examinations of Congressional witnesses including Big Pharma executives, jumped into the fray on July 6 by calling for an independent investigation of how FDA officials interact with pharma companies like Biogen.
Patients should have confidence that their treatment plans are based on science, not cozy relationships between Big Pharma and @US_FDA officials. I'm concerned the approval process for certain drugs has been compromised, so I'm urging an independent watchdog to investigate. pic.twitter.com/LgLzcIGVnN
Porter specifically points to the so-called "Project Onyx," an alleged effort by Biogen to lobby top FDA officials to get Aduhelm — a drug the company's own independent safety monitoring board for clinical trials deemed "futile — across the regulatory finish line. A report by STAT News points to multiple off-the-record and back channel meetings between Biogen and senior FDA staff who appeared determined to get Aduhelm to the market.
Aducanumab's financial and regulatory future is still up in the air. But between the skepticism of physicians and the wrath of politicians, the messiness of the process leading to this ostensible Alzheimer's breakthrough's approval is breeding some chaos.






